Project management
Our project managers have extensive experience ranging from initial first-in-human phase I studies through to large-scale international and multicentre studies. As your contact they will plan, coordinate and oversee your project and ensure that all the relevant information is made available at the right place and at the right time.
Procurement
We will source traditional packaging materials and the appropriate tools on your behalf. Thanks to our network of specialist partners, we are able to offer you supplementary services such as blinding by over-encapsulation or analytical tests.
Randomisation and unblinding
We can prepare randomisation lists for all types of study, e.g. simple or complex studies (placebo and/or competitor-controlled), titration and crossover studies also employing double-dummy methods. In addition, we will prepare emergency envelopes (code breakers) for the potential disclosure of individual patient medication should this prove necessary.
Identification and label printing
We will identify your trial drug using mono- or multilingual labels and with booklets containing any Unicode-encodable character set (including Western, Eastern European and Asian fonts). The printing and supply of labels and booklets is segregated strictly according to treatment groups, as per the specifications in the packaging plan.
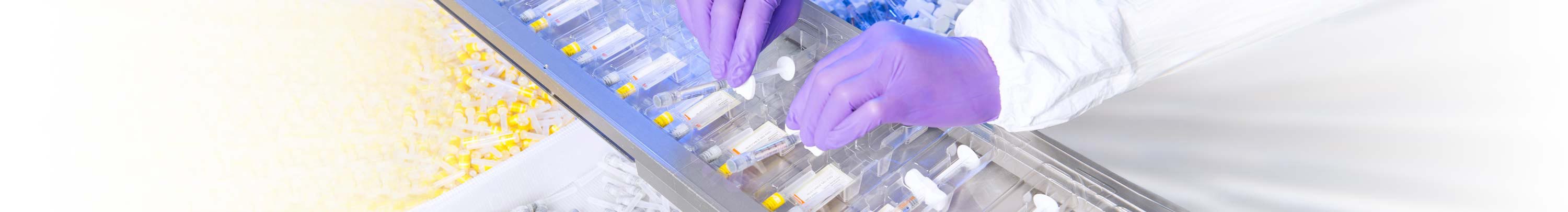
Packaging
Our premises and machinery are audited by the FDA and Swissmedic. The validated, GMP-compliant processes are also suitable for supplies of clinical trial materials to Japan. When it comes to packaging, you can rely on our experience and expertise in primary and secondary packaging, which also takes into account the special requirements that apply to clinical trial samples. We have at our disposal a large number of tools and lines for widely varying batch sizes and complexity levels for studies from phase I through to phase IV.
Quality control and EU release
Your order-specific packaging instructions will be approved by our QA/QC department. Individual work steps will be accompanied by continual in-process controls and documented in their entirety. The packaging protocols and the packaged intermediate and end products will be approved in conjunction with the issue of a corresponding GMP certificate by a qualified person. On request, we shall be pleased to guide your products through the EU release process in collaboration with partners.
Storage, distribution, returns and disposal
We will store your clinical trial materials with access restriction within the agreed temperature ranges, and undertake worldwide distribution to study centres in conjunction with selected transport logistics companies. In the case of complex IVRS-supported studies, pick-label-pack order processing is also possible. We will be delighted to support you with batch tracing or the monitoring of expiry periods. We will process, account for and administer returns of clinical trial materials and also ensure disposal in conjunction with a qualified partner.
|